Research
TBI-SPIRIT
People who experience traumatic brain injury (TBI) may encounter barriers to participation in meaningful roles, activities, and relationships for weeks to years after their injury.
The overall goal of this 5-year study supported by the National Institute of Disability, Independent Living and Rehabilitation Research (NIDILRR) is to develop an efficient assessment that can inform clinicians, family members, and researchers about challenges to community participation, monitor social participation recovery and assess their needs to connect individuals with TBI to needed resources in the community. The TBI-SPIRIT study will use advanced psychometric technologies (Item Response Theory (IRT), Computer Adaptive Testing (CAT) and Machine Learning (ML) to develop the TBI-SPIRIT profile assessment for persons with TBI that span the severity spectrum and focus on key domains that include the activities you do, such as work and leisure activities, and the relationships you have, such as friendships and romantic relationships. The TBI-SPIRIT Profile will be comprised of items developed specifically for persons with TBI that assess their unique social participation challenges. The product of this work will be a tool for use in those with TBI for future research that monitors outcomes of care. The TBI-PCAT also will be used for evaluating interventions in future clinical trials. The study will be conducted in several phases which include: (1) Interviews and Focus Groups: We will meet with individuals with TBI, their family members, and clinicians to identify important aspects of participation and develop questions for the assessment. (2) Testing and Refinement of the measure: We will administer a large number of items to approximately 500 individuals with TBI from across the country to evaluate and refine the questions on the assessment and calibrate the scoring using advanced measurement methods. We will then administer the refined measure to additional individuals with TBI to assess the reliability and validity of the measure.
Research Coordinators:
Caitlin Rajala
Camden Waterhouse
Improving Resilience in Survivors’ Experience (I RISE)
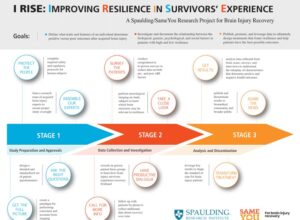
Spaulding Research Institute for Brain Injury and Concussion Recovery partnered with actress Emilia Clarke’s foundation SameYou to improve the quality of care patients receive following neurological trauma.
Thanks to SameYou’s vision and incredible generosity, we are launching a groundbreaking research study, the I RISE Research Project, to better understand the brain’s “resilience” both before and after an acquired brain injury- such as those caused by stroke, head trauma, and illness.
When it comes to the brain, peoples’ resilience (ability to thrive or bounce back after trauma) can vary dramatically even though their conditions may be very similar. Some patients with more serious injuries progress surprisingly well, and others with seemingly less complex conditions may struggle. Early-life experience and exposures, biology and genetics, injury severity, post-injury treatment, and even personal coping mechanisms like faith, beliefs, or outlook, all appear to play an important role.
Goals of I RISE:
- Unravel how these biological, psychological, and social factors interact to influence patients’ course of recovery and their response to interventions.
- Leverage our data to design treatment strategies that promote resilience and help patients have the best possible outcomes.
Research Coordinator: Julia Birch
Rehabilitation and COVID-19 Recovery (RECOVR) Study
Very little is known about the long term impact of COVID-19 illness and recovery on physical and emotional health, or the rehabilitation care needs of hospitalized COVID-19 patients once they are discharged from acute care.
The TBIMS is a multi-center program that aims to monitor and improve long term outcomes for individuals with traumatic brain injury through enrollment in a national database, multi-center “module” projects, and single center studies. Spaulding has become a national leader in TBI research and rehabilitation under the Model Systems program as a result of excellent clinical care, research contributions, and educational efforts. The Rehabilitation and COVID-19 Recovery (RECOVR) Study aims to increase our understanding of the recovery trajectory and the long-term health outcomes after inpatient acute care hospitalization for COVID-19 related illness. Our team is prospectively collecting longitudinal outcome data from study participants in physical, cognitive, psychological, and social participation domains. We aim to assess the relationship between acute and post-acute care interventions and long-term functional outcomes, and to document patient-reported health and quality of life up to a year after their acute admission to the hospital. We hope to understand the long-term physical, cognitive, and psychological impact of severe COVID-19 illness.
Research Coordinator: Lydia Borsi
Traumatic Brain Injury Model System (TBIMS)
The Spaulding-Harvard Traumatic Brain Injury Model System (SH-TBIMS), led by Dr. Joseph T. Giacino, was selected by the National Institute of Disability, Independent Living, and Rehabilitation Research (NIDILRR) to receive federal funding as a TBIMS center.
TBIMS research is responsible for almost 500 peer-reviewed publications and has facilitated the development of TBI medical care guidelines, comprehensive diagnostic procedures, rehabilitation approaches, and more. Within the 16 TBIMS centers throughout the nation, exceptional rehabilitation additionally serves to improve injury treatment and outcomes for future patients.
Research Coordinator: Ally Sterling
TBIMS National Database
Some patients who enter the Brain Injury Program at Spaulding Rehabilitation Hospital Boston and Spaulding Hospital Cambridge are eligible to anonymously contribute their data to the TBI Model System national database. The objective of this project is to build a repository of data that will aid future TBI patients, as well as their families and clinicians.
Researchers from across the nation conduct studies using the information housed in this database. The TBI Model System National Database is over 30 years old and houses information from over 18,000 individuals. Information collected for the database includes medical history from before the injury, as well as details of the patient’s hospital course. Researchers obtain information through medical records, a questionnaire, and a brief bedside test. Researchers also conduct follow-up phone interviews at 1, 2, 5, and every 5 years after post-injury to follow patients for life. The information collected remains confidential. This study presents a great opportunity for patients to help future patients and clinicians with minimal effort and risk to themselves.
Click here to see a list of other sites currently contributing to the TBI Model System National Database.
Principal Investigator:
Joseph T. Giacino, PhD
Development, validation and feasibility of the Coma Recovery Scale- Revised for Accelerated Standardized Testing (CRSR-FAST)
The CRSR-FAST will enable longitudinal assessment across the continuum of recovery, bridging the communication divide between the acute care and rehabilitation settings.
Standardized Testing (CRSR-FAST)
In light of the high incidence of diagnostic error among patients with disorders of consciousness (DoC) (estimated to be 30-40%), and the risk of premature withdrawal of life-sustaining treatment, we are testing the validity of an abbreviated version of the Coma Recovery Scale-Revised (CRS-R) for use in the intensive care unit, the CRSR For Accelerated Standardized Testing (CRSR-FAST). The CRSR-FAST will provide clinicians with a brief, standardized assessment measure that can accurately determine level of consciousness.
Principal Investigator:
Yelena Bodien, PhD
Partnering with Caregivers to Increase Knowledge of the Post-Acute Phase of Recovery from Severe TBI: Development and validation of the “Post-Acute Survey on Severe Disability after TBI (PASSD-TBI)”
The PASSD-TBI will provide an efficient means of obtaining long-term outcome data on persons with prolonged disorders of consciousness, identify favorable and unfavorable influences on caregiving activities and increase knowledge of caregiver burden.
We are leading a multi-center study, in collaboration with the Indiana TBIMS, TIRR TBIMS, Tampa VA Polytrauma Rehabilitation Center (PRC), Richmond VA PRC, and San Antonio VA PRC, that will develop and test the feasibility of a telephone-based structured caregiver interview, the “Post-Acute Survey on Severe Disability after TBI (PASSD-TBI).”
If the results of the pilot study are favorable, the PASSD-TBI will be nominated for inclusion in the TBIMS National Database.
Principal Investigator:
Joseph T. Giacino, PhD
Trajectories of Cognitive Functioning Years after TBI
The aim of this study is to characterize patterns of cognitive function over time among individuals who are 2-7 years post-injury.
Participants will be administered the Brief Test of Adult Cognition by Telephone (BTACT) to assess one group of TBIMS participants 5, 6, and 7 years post-injury, and another group 2, 3, and 4 years post-injury. This study will allow the TBIMS, for the first time, to contribute to the advancement of knowledge regarding long-term cognitive functioning after traumatic brain injury. This project is led by the New York TBIMS program.
Principal Investigator:
Kristen Dams-O’Connor, PhD (New York TBIMS)
SH-TBIMS Principal Investigator:
Joseph T. Giacino, PhD
Development and Assessment of Crosswalks in the TBIMS Database
Using data from the TBIMS National Database, this study will evaluate various procedures for creating crosswalks between the FIM tool and the Continuity Assessment Record and Evaluation (CARE) Tool Item Set, as well as evaluate the existing crosswalk between the Patient Health Questionnaire (PHQ-9) and the Traumatic Brain Injury Quality of Life (TBI-QOL) Depression Short Form and between the Generalized Anxiety Disorder 7-item (GAD-7) scale and the TBI-QOL Anxiety Short Form.
This project is led by the Rocky Mountain Regional Brain Injury System.
Principal Investigator:
Dave Mellick, MA (Rocky Mountain Regional Brain Injury System)
SH-TBIMS Principal Investigator:
Therese O’Neil-Pirozzi, ScD, CCC-SLP
TBIMS Multicenter Modular Projects
TBIMS Multicenter Modular Projects Include:
- “Internet Use and Online Social Participation among Individuals with TBI”
- “Cognitive Testing (BTACT) in the TBI Model Systems”
- “Statins and Outcome After TBI: An Observational Study”
- “Development of an Extended Measure of Global Function to Support Clinical Trials Originating in Acute Rehabilitation”
- “Understanding Cause of Death in the TBI Model Systems”
For more information about the Spaulding-Harvard TBI Model System, please contact Dr. Michael Bergin at:
Phone: (617) 952-6310
Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI)

The TRACK-TBI Consortium is a partnership of top tier academic and Level 1 Trauma Centers across the United States.
Launched in 2009, the TRACK-TBI Pilot study (NIH RC2 NS069409) has:
- Validated the NINDS TBI Common Data Elements.
- Collected detailed clinical data on 650 subjects across the injury spectrum, along with CT/MRI imaging, blood biospecimens, and detailed outcomes.
- Built an infrastructure of integrated clinical databases, imaging repositories, biosample repositories, and coordinated multisite/multidisciplinary expertise.
- Received ongoing financial and in-kind support from patient advocacy foundations and private industry partners in the neuroimaging, pharmaceutical, device, and data management and analytic spaces
- TRACK-TBI expanded to 11 sites with the launch of the TRACK-TBI U01 phase in 2013 (NINDS U01 NS086090). 7 new institutions joined the consortium during the U01 phase (2017), resulting in a total of 18 enrolling clinical sites with additional sites providing analytic support.
Goals of TRACK-TBI:
-
- Describe the natural history of TBI.
- Establish precise methods for TBI diagnosis and prognosis.
- Refine outcome assessments.
- Compare effectiveness and costs of TBI care.
The extensive protocols empower rich, multidimensional characterization of the clinical, neuroimaging, and blood-based biomarker features of TBI. TRACK-TBI has amassed the world’s largest and most comprehensive serial collection of standardized TBI neuroimaging (CT and MRI), using structural, functional, and diffusion phantoms for quantitative imaging, and developed automated pipelines for imaging quality assurance. The TRACK-TBI study followed participants longitudinally for one year from time of injury.
With the close of U01 funding in 2018, continued enrollment into the TRACK-TBI protocol was supported by an unrestricted gift from the National Football League (i.e., “Post-U01 cohort”).
As of July 2020, TRACK-TBI U01+Post-U01 has enrolled >3050 TBI subjects and >350 orthopedic control subjects.
Research Coordinator: Kelsey Goostrey
TRACK-TBI Longitudinal (TRACK-TBI LONG)

TRACK-TBI LONG, launched in May 2019, extends follow-up of the deeply phenotyped TRACK-TBI cohort into the chronic phase.
This is the first and largest study of incident TBI to couple comprehensive multi-year clinical trajectories with advanced neuroimaging and proteomic biomarkers.
Goals of TRACK-TBI LONG:
- Connect with participants enrolled in the TRACK-TBI-II study to assess their functional status two or more years after their original study injury.
- Elucidate TBI’s natural history.
- Identify individuals most at risk for unfavorable outcomes.
- Initiate development of diagnostic, prognostic, and therapeutic/ management tools for this heterogeneous condition.
TRACK-TBI LONG expands upon the original TRACK-TBI study protocol. TBI and Control participants from the TRACK-TBI U01 study as well as participants enrolled under the Post-U01 phase of the study will be eligible for up to 3 annual TRACK-TBI LONG Telephone Assessments.
TRACK-TBI LONG is supported by funding through a competitive grant from the National Football League Scientific Advisory Board and the National Institute of Neurological Disorders and Stroke (Post-UO1 participants).
Research Coordinator: Kelsey Goostrey
Clinical Validation of Serum Neurofilament Light as a Biomarker of Traumatic Axonal Injury (TRACK-TBI BIO)

Traumatic brain injury (TBI) is a leading cause of mortality and morbidity and a strong risk factor for neurodegenerative disorders later in life.
The absence of validated biomarkers in the neurotrauma field prevents drug development, so there are no disease-modifying therapies that successfully limit the burden of TBI.
Traumatic axonal injury (TAI) underlies the most disabling consequences of TBI. Recent breakthroughs in pre-clinical models indicate that novel therapeutic interventions are effective in promoting resilience of injured axons and improving neurologic outcome after experimental TBI.
Successful translation of these therapies will require prognostic biomarkers that measure TAI in individual patients and pharmacodynamic biomarkers that measure treatment efficacy. Currently, the best biomarker for TAI is fractional anisotropy (FA) and mean diffusivity (MD) of white matter tracts, measured using diffusion tensor imaging (DTI) MRI. This technique is poorly suited for dynamic longitudinal assessments and measures the end-result of axonal degeneration, rather than earlier neurodegenerative stages. The recent ability to assay axonal proteins in peripheral blood has made it possible to assess TAI rapidly, inexpensively, and longitudinally.
Goal of TRACK-TBI BIO:
- Clinically validate the axonal protein neurofilament light chain (NfL) as a prognostic biomarker of TAI.
- The TRACK-TBI Biomarker Project NF-L (TRACK-TBI BIO NF-L) extends the follow-up periods for TRACK-TBI participants from 1 to 5 years. The clinical, imaging, and biomarker data already collected in these subjects will allow for the identification of risk factors, co-morbidities, and prognostic biomarkers of TBI. The extension of study follow-up will help determine negative neurological and psychological outcomes of individuals who experienced a TBI compared to healthy controls.
- A secondary objective is to validate serum NF-L as a biomarker of traumatic axonal injury, and obtain data that will allow us to submit a Qualification Plan to the Center for Drug Evaluation and Research at FDA for qualification of serum- NF-L as a Drug Development Tool.
- The TRACK-TBI Biomarker Project Tau/pTau (TRACK-TBI BIO Tau/PTau) aims to validate Tau and P-Tau as prognostic biomarkers for complicated mild TBI.
Research Coordinator: Kelsey Goostrey
TRACK-TBI Epileptogenesis Project (TRACK-TBI EPI)

Post-traumatic epilepsy (PTE) is a common TBI complication, occurring in up to 20% of civilian patients and as many as 50% of military service members who suffer severe brain trauma.
Epilepsy resulting from brain trauma is difficult to control with medical therapy and accounts for 5% of patients referred to specialized epilepsy centers.
PTE can arise from TBI of any severity and through a variety of mechanisms, which may co-exist within a single patient. Epileptogenesis can result from penetrating trauma or focal contusions known as focal brain injury. Closed head injury can also produce diffuse injury, with shearing of axons and blood vessels, diffuse edema and ischemia, and secondary cellular damage through the release of inflammatory mediators.
The clinical features of epilepsy such as seizure frequency and severity, prevalence of associated co-morbidities, and responsiveness to therapy, may differ. Additionally, there is variation in neurophysiologic and imaging features of epileptogenicity. Indeed, a sophisticated understanding of the subtypes of epilepsy resulting from brain trauma is required to successfully develop anti-epileptogenic therapies.
Goal of TBI-TRACK EPI:
Determine how PTE contributes to negative neurological and psychological outcomes of individuals who experienced a TBI through comparisons to patients with TBI but without PTE.
Funded in March 2019, TRACK-TBI Epileptogenesis Project (TRACK-TBI EPI) extends the follow-up period of the TRACK-TBI cohort up to 5 years after injury to identify patients who developed PTE. The TRACK-TBI NINDS PTE Screening Questionnaire identifies participants who screen positive for PTE and consent them to undergo a detailed clinical evaluation. Data collected via a combination of follow up phone screening and in-person visits assesses the relationship between TBI severity and epileptogenesis as the primary endpoint. These data will provide the first comprehensive longitudinal phenotyping of subjects with PTE from the moment of TBI through their epilepsy diagnosis and treatment.
TRACK-TBI EPI is supported by the U.S. Army Medical research and Development Command.
Research Coordinator: Kelsey Goostrey
Abbott i-STAT
The i-Stat TBI Plasma Test is a biomarker-based assay designed to objectively assess the need for CT.
TRACK-TBI has partnered with Abbott to test its prototype TBI point-of-care device. The i-STAT handheld blood analyzer and blood test can be used at the patient’s bedside to detect elevated levels of TBI biomarkers like GFAP and UCHL-1.
This is the first rapid handheld TBI blood test, which will help clinicians assess individuals with suspected mild TBIs, including concussions. Tests results are available within 15 minutes after plasma is placed in the test cartridge. The technology could help detect brain injury even if CT scan is normal.
The study aims to enroll ~1100 subjects over 2 years across the Network sites. Six sites are now enrolling participants for this study: UCSF, University of Pittsburgh Medical Center, Baylor College of Medicine, UT Austin, University of Pennsylvania, and Medical College of Wisconsin.
Research Coordinator: Kelsey Goostrey
TBI Endpoints Development Initiative
In collaboration with the FDA, TBI Endpoints Development (TED) researchers will examine existing datasets from thousands of athletes, soldiers and the broader population.
This study will identify which current measures are most effective, and validate these measures or “endpoints” of brain injury and recovery. The TED team is comprised of leading academic clinician-scientists, industry leaders in biotechnology and imaging technology, patient advocacy organizations, and philanthropies.
TBI is a multifaceted condition, and as of 2015, no drug or device has been approved by the U.S. Food and Drug Administration (FDA) to treat acute TBI.
The lead site is the University of California, San Francisco.
First among TED’s objectives (Stage I) is to establish a TED Metadataset consisting of integrated clinical outcomes, and imaging, proteomic, and genomic data, from ongoing and legacy TBI studies across civilian, military, and sports cohorts. This highly detailed and multi-scalar repository will be continuously curated and analyzed using conventional and novel methodologies. TED researchers aim to identify and validate meaningful TBI endpoints, as well as structural abnormalities that may be predictive of outcomes, making strides toward a new taxonomy for TBI.
Among other objectives (Stage II) TED will support the selection, funding, and oversight of four Seed Projects focused on validation of clinical outcome assessments (COAs) and blood-based and neuroimaging biomarkers. Creating a range of validated COAs, biomarkers, and devices will:
-
- Permit more accurate disease/condition diagnosis
- Identify patient subpopulations likely to benefit from therapy/intervention
- Provide refined outcome assessments to confirm efficacy
Evidence-Based Clinical Outcome Assessment Platform (EB-COP)
The TED Government Steering Committee selected Spaulding Rehabilitation Hospital’s proposal for Evidence-Based Clinical Outcome Assessment Platform (EB-COP) as one of four awardees to receive funding for 1-year projects commencing in 2016.
The primary aim of EB-COP was to design, build, and pilot-test a semi-automated assessment platform to enable the efficient, transparent, systematic and context of use (COU)-specific grading of COAs to inform selection of suitable candidate measures for submission to the FDA. The Platform will also be disseminated to Investigators as a tool to help foster validation of existing COAs.
The EB-COP evaluates the strength of COAs using a six-step process that begins with the guided specification of the COU. The COA is ultimately graded using a four-tiered recommendation scheme based on the number of mandatory quality indicators appraised as adequate.
The team has pilot tested the EB-COP on the Glasgow Outcome Scale-Extended for the detection of treatment effects in patients with moderate/severe TBI of subacute duration. Results from this pilot test were presented to the Government Steering Committee on March 17, 2017 and at the Second TED Consensus Conference held on the NIH campus on April 4-5th, 2017. In collaboration with the American Academy of Neurology, the investigators are finalizing a Qualtrics software platform designed to semi-automate the COA review and grading process.
Read a complete description of the methodology, development, and evaluation of the findings from the EB-COP pilot-testing published here. Funded by the Department of Defense.
Primary Investigator: Dr. Joseph Giacino, PhD (jgiacino@mgh.harvard.edu)
Coma Recovery Scale- Revised

Coma Recovery Scale- Revised:
https://www.sralab.org/rehabilitation-measures/coma-recovery-scale-revised
Disorders of Consciousness Guidelines
Disorders of Consciousness Guidelines:
https://www.aan.com/Guidelines/Home/GuidelineDetail/926
Full-Length Version: https://www.aan.com/Guidelines/Home/GetGuidelineContent/928
Presentation Slides: https://www.aan.com/Guidelines/Home/GetGuidelineContent/931
Ethics Commentary: https://www.aan.com/Guidelines/Home/GetGuidelineContent/932
Podcast: https://www.aan.com/Guidelines/Home/GetGuidelineContent/937
Press Release: https://www.aan.com/Guidelines/Home/GetGuidelineContent/933
Consumer Summaries:
Clinician Summary: https://www.aan.com/Guidelines/Home/GetGuidelineContent/929
Family/ Caregiver Summary: https://www.aan.com/Guidelines/Home/GetGuidelineContent/930
Family Caregiver Summary (Spanish Version): https://www.aan.com/Guidelines/Home/GetGuidelineContent/951
Rehabilitation Outcomes Center at Spaulding (ROCS
Rehabilitation Outcomes Center at Spaulding (ROCS):
https://spauldingrehab.org/research/programs-labs/rehabilitation-outcomes-center